full electron configuration for br|Electronic configuration of bromine ? : Tuguegarao The total number of electrons in bromine is thirty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in bromine in . Tingnan ang higit pa Previous Results. You can view other recent Lotto results below. Select a specific date to get more details about the draw. If you have won a prize, you have 180 days from the date of the draw to claim it.
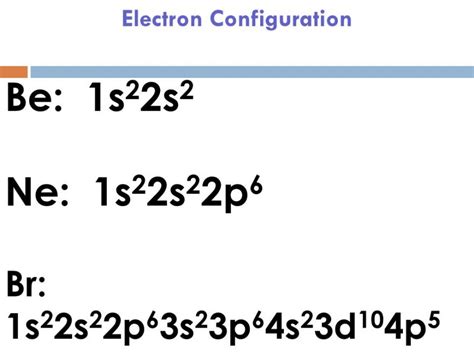
full electron configuration for br,Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of bromine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. In the bromine ground-state electron configuration, the last five electrons of the 4p orbital are located in the . Tingnan ang higit paThe total number of electrons in bromine is thirty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in bromine in . Tingnan ang higit pa
full electron configuration for br Electronic configuration of bromine ? Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit paThe electron configuration of bromine shows that the last shell of bromine has seven electrons. Therefore, the valence electrons . Tingnan ang higit pa In this video we will write the electron configuration for Br-, the Bromide ion. We’ll also look at why Bromine forms a 1- ion and how the electron configura. Bromine Orbital Diagram. In this article today we are going to tell you about the electron configuration of Bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of the same. Please .The electronic configuration for $\ce{Br-}$ is: $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ Because it have one more electron than bromine, which ends its . The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom.Bromine electron configuration. ← Electronic configurations of elements. Br (Bromine) is an element with position number 35 in the periodic table. Located in the IV period. .

Electron configuration of Bromine is [Ar] 3d10 4s2 4p5. Possible oxidation states are +1,3,5/-1. Electron Configuration. The periodic table is a tabular display of the . Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.⬆. Get the facts about element Bromine (Br) [35] from the periodic table. Find physical data, electron configuration, chemical properties, aggregation states, isotope data (including . The electron configuration of Bromine is 1s22s22p63s23p64s23d104p5. This can be shortened to [Ar]4s23d104p5. Explanation: Use a chart such as the one . The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be .Reduced electronic configuration Br: [Ar] 3d 10 4s 2 4p 5. Below is the electronic diagram of the Bromine atom Distribution of electrons over energy levels in the Br atom 1-st level (K): 2 2-st level (L): 8 3-st level (M): 18 4-st .
The Br atom has 4s 2 3d 10 4p 5 as the electron configuration. Therefore, Br has 1 unpaired electron. Answer (c): The B atom has 2s 2 2p 1 as the electron configuration. Because it has one unpaired electron, it is paramagnetic. Answer (d): The F-ion has 2s 2 2p 6 has the electron configuration. Because it has no unpaired electrons, it is . In addition to forming Fe 2+ ions, iron atoms are also capable of forming Fe 3+ ions. Extra stability is gained by electron configurations that have exactly half-filled d orbitals. The third electron that is removed from an iron atom to form the Fe 3+ ion is removed from the d orbital giving this iron ion a d orbital that is exactly half-filled. The .
Bromine electron configuration notation. The electronic configuration notation of Br is represented by the notation; Br : Ar 18 4s 2 3d 10 4p 5. Bromine unabbreviated electron configuration. Br: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. When there is no noble gas configuration for the starting electrons, this is referred to as an .Bromine(Br): Bromine is a p-block element having an atomic number 35. Bromine belongs to group 17 which is known as halogens. Electronic configuration of Group 17: The elements of group 17 have seven electrons in their outermost shell. So, the valence electronic configuration of group 17 is n s 2 n p 5. Electronic configuration of Bromine: Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure.The chemical symbol for Bromine is Br. Electron Configuration and Oxidation States of Bromine. Electron configuration of Bromine is [Ar] 3d10 4s2 4p5. Possible oxidation states are +1,3,5/-1. Electron .Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written (here is an explanation why). Therefore we have (still incorrect) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9 4s 2. Correct Electron Configuration for Copper (Cu)
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
Electronic configuration of bromine ? Write the electron configuration for Br−. Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on.full electron configuration for br 1s^2 2s^2 2p^6 3s^2 3p^6 Based on the periodic table, the atomic number (Z of chlorine is 17. Since the atomic number is always equal to the number of protons or Z = number of protons and in ground state (no charge), the number of protons is equal to the number of electrons, then Z = number of protons = number of electrons (ground state) .

Complete the electron configuration for Br. electron configuration: [Ar] Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on. The noble gas configuration for bromine is : [Ar]3d^(10)4s^(2)4p^(5) The previous noble gas is argon which has the electron configuration of : 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6) We call this the argon core. It's a handy way of writing out electron structures without writing all the inner electron shells.The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule. When writing an electron configuration, you have to write serially. Ruthenium ion(Ru 3+) electron configuration. The ground state electron configuration of ruthenium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 7 5s 1. This electron configuration shows that the last shell of ruthenium has an electron and the d-orbital .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon . We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also acceptable. The ground-state electron configuration refers to the most stable arrangement of electrons around the nucleus of an atom. Here are the ground-state electron configurations for the following atoms: Br (Bromine): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵; Mg (Magnesium): 1s² 2s² 2p⁶ 3s²; Se (Selenium): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² .
full electron configuration for br|Electronic configuration of bromine ?
PH0 · How can I figure out the electron configuration of Br
PH1 · Electronic configuration of bromine ?
PH2 · Electron configuration for Bromine (element 35). Orbital diagram
PH3 · Electron Configuration Calculator
PH4 · Complete Electron Configuration for Bromine (Br, Br
PH5 · Bromine electron configuration
PH6 · Bromine Electron Configuration (Br) with Orbital Diagram
PH7 · Bromine (Br) [35] — Chemical Element — Periodic Table
PH8 · Bromine
PH9 · Br